Crystallography is the study of how atoms and molecules arrange themselves into regular, repeating patterns called crystals. It helps you understand the internal structure that gives crystals their unique shapes and properties. Crystals form through specific growth conditions, influenced by atomic bonds and symmetry. Techniques like X-ray diffraction are used to analyze these structures in detail. If you’re curious, exploring further reveals how this science shapes materials and discoveries worldwide.
Key Takeaways
- Crystallography studies the internal atomic structure and symmetry of crystals using techniques like X-ray diffraction.
- Atoms and molecules form the basic building blocks, with bonding types influencing crystal stability and properties.
- Crystal formation involves atoms assembling in repeating geometric patterns under specific environmental conditions.
- Symmetry, including point groups and translational patterns, classifies crystals and explains their shapes and behaviors.
- Analytical methods such as X-ray diffraction, electron microscopy, and neutron scattering reveal detailed crystal structures.

AugustknowU 50x50mm Interference Diffraction Grating Kit Including Multi-Slit, Round Hole, Cross Line and Composite Patterns for Optical Lab Use (Single Slit)
💡[Interference Diffraction Grating Set (50x50mm)] Includes 1 x single-slit grid (0.05, 0.1, 0.2mm seam width), 1 x 1"…
As an affiliate, we earn on qualifying purchases.
As an affiliate, we earn on qualifying purchases.
What Is Crystallography?

Have you ever wondered how scientists determine the internal structure of crystals? Crystallography is the field that explores this. It involves studying the arrangement of atoms and molecules within a crystal, which is often revealed through molecular symmetry. This symmetry helps scientists classify crystals and predict their properties. However, real crystals aren’t perfect; crystal defects like vacancies or dislocations can disrupt symmetry and affect their behavior. By analyzing how these defects influence molecular symmetry, researchers gain insights into a crystal’s stability and growth patterns. Crystallography combines techniques like X-ray diffraction and microscopy to uncover the hidden atomic architecture. Understanding how molecular symmetry and crystal defects interact is essential for deciphering a crystal’s structure and tailoring materials for specific applications. Crystal defects can significantly influence physical and chemical properties, making their study vital in material science.

LINKTOR Chemistry Molecular Model Kit (444 Pieces), Student or Teacher Set for Organic and Inorganic Chemistry Learning, Motivate Enthusiasm for Learning and Raising Space Imagination, A Fullerene Set
FOR BASIC TEACHING TO ADVANCED SCIENCE: 444 pieces molecular model kit, including 136 atoms, 158 bonds and 150…
As an affiliate, we earn on qualifying purchases.
As an affiliate, we earn on qualifying purchases.
The Building Blocks: Atoms and Molecules
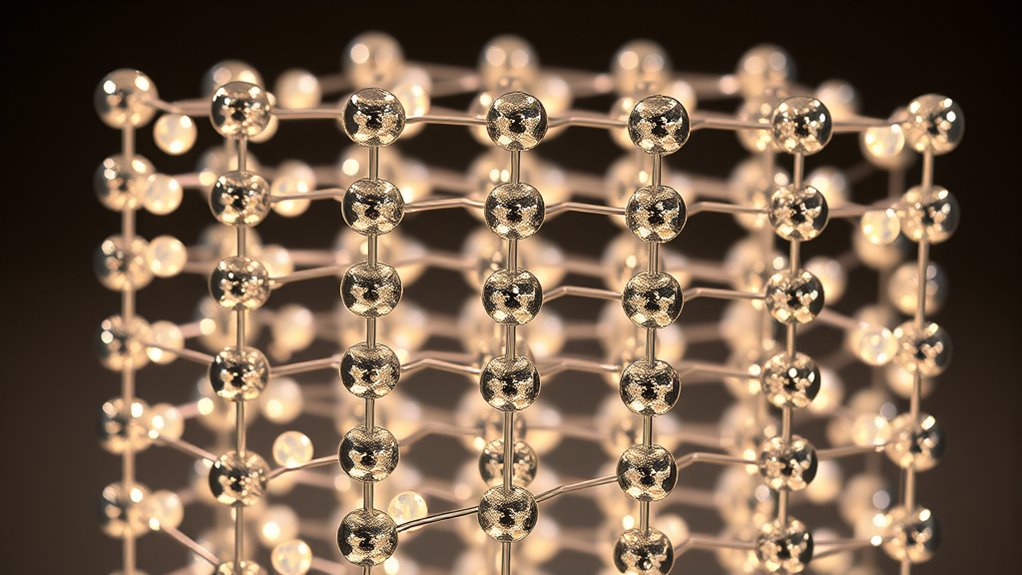
Atoms and molecules are the fundamental building blocks of all crystalline structures, forming the basis for their physical properties. You can think of atomic bonding as the glue that holds atoms together within a crystal, determining how they interact and arrange themselves. Molecular geometry describes the shape and spatial arrangement of atoms within a molecule, influencing how these molecules pack in the crystal lattice. By understanding atomic bonding, you gain insight into the strength and type of connections—covalent, ionic, or metallic—that define crystal properties. Recognizing molecular geometry helps you visualize how molecules fit together in space, affecting crystal symmetry and stability. The type of atomic bonding significantly influences the overall stability and behavior of the crystalline material. These core concepts are essential for understanding how atoms and molecules form the ordered patterns characteristic of crystalline materials. Additionally, the arrangement of atoms within the crystal lattice impacts physical properties such as hardness, melting point, and optical characteristics. For example, the bonding type can determine whether a crystal is brittle or malleable.

Snatoms X by Veritasium – The Ultimate Magnetic Molecular Modeling Set | v2.0 Stronger Magnets | Engaging Science Education Kit for STEM Learning | Intro to Atoms, Molecules, Bonding, Chemistry
Innovative Design – v2.0 features improved magnets allowing stronger magnetic bonding than ever before. The unique magnetic interlocking…
As an affiliate, we earn on qualifying purchases.
As an affiliate, we earn on qualifying purchases.
How Crystals Form

Ever wondered how crystals develop their orderly structure? Crystal growth begins when atoms or molecules in a solution, melt, or vapor come together and arrange themselves in a repeating pattern. This process is driven by mineral formation, where specific conditions like temperature, pressure, and concentration influence how quickly and perfectly crystals form. As these particles bond in a regular, geometric pattern, the crystal structure takes shape. During growth, crystals often develop facets and unique shapes that reflect their internal symmetry. The more stable the environment, the more uniform and well-defined the crystals become. Factors such as environmental stability significantly impact crystal formation and quality. Additionally, nucleation sites play a crucial role in determining where and how crystals begin to form, affecting the overall quality and size of the crystals. Understanding how crystals form helps explain the natural beauty of mineral specimens and lays the foundation for applications in technology, medicine, and industry. Furthermore, controlled conditions are essential for optimizing crystal growth in laboratory and industrial settings.

Crystal structure determination in the scanning electron microscope: Fundamental and experimental problems
As an affiliate, we earn on qualifying purchases.
As an affiliate, we earn on qualifying purchases.
The Symmetry of Crystals

Once crystals start to form, their internal arrangement follows specific patterns that give each crystal its unique shape. This pattern is governed by symmetry, which describes how parts of the crystal relate to each other. You’ll see point group symmetries, which include rotations, reflections, and inversions that leave the crystal unchanged. These symmetries help classify crystals into different symmetry groups. Additionally, translational symmetry plays a key role, meaning the pattern repeats regularly as you move through the crystal lattice. Together, point group symmetries and translational symmetry define the overall symmetry of a crystal. Understanding these concepts helps you recognize the ordered, repetitive nature of crystal structures and explains why crystals have such consistent shapes and properties.
Crystal Lattices and Unit Cells

A crystal lattice is a three-dimensional, repeating arrangement of points that represents the positions of atoms, ions, or molecules within a crystal. The smallest repeating unit is the unit cell, which defines the entire lattice’s structure. By analyzing the unit cell, you can classify it into categories like primitive, body-centered, or face-centered based on its symmetry. Determining lattice parameters—edge lengths and angles—helps you understand the unit cell’s shape and size, essential for crystal characterization. Accurate lattice parameter determination is vital for identifying crystal systems and understanding their properties. Here’s a quick overview:
| Unit Cell Classification | Description |
|---|---|
| Primitive | Atoms only at corners |
| Body-centered | Additional atom at the center |
| Face-centered | Atoms at face centers |
| Edge-centered | Atoms at edge centers |
Common Types of Crystals

Understanding the different types of crystals is essential for grasping their unique properties and behaviors. Crystals come in various shapes, known as crystal shapes, which result from their internal atomic arrangements. These shapes influence how crystals grow and interact with their environment. Common types include cubic, hexagonal, tetragonal, orthorhombic, monoclinic, and triclinic forms. Each shape aligns with specific mineral classifications, helping geologists identify minerals quickly. For example, halite forms cubic crystals, while quartz often appears as hexagonal prisms. Recognizing these crystal shapes allows you to understand a mineral’s structure and stability. By studying the common types of crystals, you gain insight into how minerals are classified and how their internal arrangements dictate their external forms.
Techniques Used in Crystallography

You’ll explore how X-ray diffraction methods reveal the atomic structure of crystals, providing detailed insights. Electron microscopy techniques allow you to visualize surfaces and internal features at high resolution. Neutron scattering applications help you understand magnetic properties and light elements within materials, completing the toolkit of crystallography techniques. Integrating high-resolution imaging methods enables a comprehensive analysis of crystal structures at the atomic level. Additionally, understanding the offensive security techniques used by ethical hackers can inform better security measures in sensitive research environments. Techniques such as computational modeling further enhance the understanding of complex crystal behaviors and properties. Moreover, employing neutron scattering can uncover subtle magnetic phenomena that are otherwise difficult to detect.
X-ray Diffraction Methods
Have you ever wondered how scientists determine the precise 3D arrangement of atoms within a crystal? X-ray diffraction methods are key. When crystals grow, they form a regular, repeating pattern that affects how X-rays scatter. You direct X-ray beams at the crystal, and the atoms cause the rays to diffract, creating unique diffraction patterns. These patterns contain information about the internal structure, revealing the positions of atoms. By analyzing the diffraction data, scientists can construct detailed models of the crystal’s atomic layout. This technique is highly accurate and widely used to solve complex structures. Essentially, X-ray diffraction transforms the diffraction patterns into a map of where every atom lies, giving you a clear picture of the crystal’s internal architecture. Energy monitoring features in appliances highlight the importance of understanding efficient data collection and analysis methods in scientific research. A thorough understanding of crystal symmetry also plays a crucial role in interpreting diffraction data accurately. Additionally, advancements in data processing algorithms have significantly improved the speed and precision of structure determination.
Electron Microscopy Techniques
Building on X-ray diffraction methods, electron microscopy offers another powerful approach to studying crystal structures at the atomic level. In this technique, an electron beam passes through a thin specimen in a transmission electron microscope (TEM), revealing detailed images of atomic arrangements. You can directly observe the internal structure of crystals with high resolution, identifying defects or lattice patterns. The electron beam interacts strongly with the sample, providing contrast based on atomic number differences. This method allows you to analyze crystals that are too small for traditional X-ray techniques. By capturing transmission electron micrographs, you gain insights into the material’s atomic organization. Additionally, electron microscopy can be used to detect harmful pollutants within crystal structures, which is essential for materials research. The use of advanced imaging techniques enhances the clarity and detail of the observed structures, making electron microscopy an indispensable tool in crystallography. Furthermore, improvements in sample preparation have expanded the range of materials that can be effectively studied using electron microscopy. Incorporating automation and digital technologies has also improved the efficiency and accuracy of data collection and analysis. Electron microscopy therefore complements X-ray methods, enabling you to investigate crystallography with exceptional detail and accuracy.
Neutron Scattering Applications
How can neutron scattering techniques enhance your understanding of crystal structures? Neutron applications in crystallography allow you to probe atomic arrangements with high sensitivity, especially for light elements like hydrogen. Unlike X-ray methods, neutron scattering techniques provide detailed insights into both atomic positions and magnetic structures. You can analyze complex biological molecules, materials with subtle disorder, or hydrogen-rich compounds effectively. Neutron applications also enable you to study crystal dynamics and temperature-dependent behaviors, revealing information inaccessible through other methods. Additionally, space utilization is optimized because neutron techniques can be performed with minimal sample preparation and reduced damage. For example, neutron scattering is particularly valuable in studying magnetic structures, which are difficult to analyze using other techniques. Moreover, neutron scattering allows for the investigation of dynamic processes within crystals, offering a deeper understanding of their behavior under various conditions. This technique is especially effective in examining hydrogen bonding, contributing to a comprehensive understanding of molecular interactions. By utilizing neutron scattering techniques, you gain a powerful tool to complement X-ray data, deepening your understanding of a material’s fundamental structure and properties. This approach broadens your ability to investigate materials in their native or functional states, offering a more extensive view of crystallography.
X-ray Diffraction and Its Role
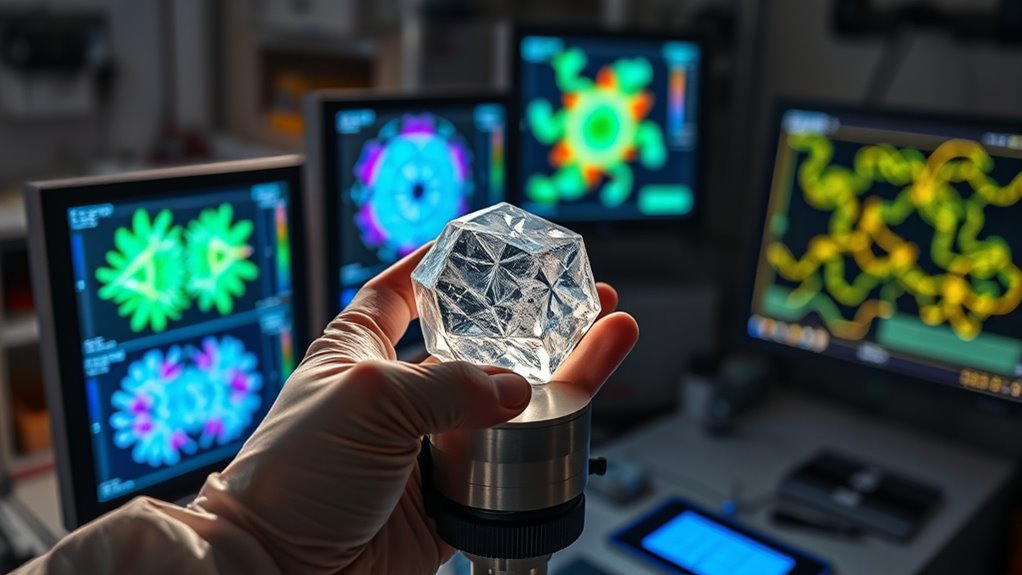
X-ray diffraction reveals the atomic structure of crystals by analyzing how X-rays scatter when they hit a material. This technique helps you understand the internal arrangement of atoms, which is essential for identifying and characterizing materials. Its applications range from developing new materials to quality control in manufacturing. Additionally, understanding the crystallography process is crucial for interpreting diffraction patterns accurately. Gaining knowledge of current advancements in crystallography can further improve the precision of material analysis.
How X-ray Diffraction Works
Ever wondered how scientists reveal the internal structure of crystals? X-ray diffraction works by directing X-rays at a crystal, causing them to scatter and produce diffraction patterns. These patterns depend on the crystal’s orientation and atomic arrangement. As X-rays hit different planes within the crystal, they diffract at specific angles, revealing information about the crystal’s internal structure. The diffraction patterns are like fingerprints, unique to each crystal’s atomic layout. Here’s a quick look at how crystal orientation affects diffraction:
| Crystal Orientation | Diffraction Pattern | Atomic Arrangement |
|---|---|---|
| Parallel to X-ray beam | Sharp spots | Regular lattice |
| Slightly tilted | Shifted spots | Slightly altered |
| Random | Blurred pattern | Disordered |
Applications in Material Analysis
Understanding the internal structure of materials is essential for developing new substances and improving existing ones. X-ray diffraction plays a vital role in material analysis by revealing details about crystal growth and identifying defects. When you analyze diffraction patterns, you can determine how crystals form and grow, which helps optimize manufacturing processes. Additionally, XRD enables you to detect imperfections and defects within the crystal lattice, critical for assessing material quality and performance. This information guides improvements in material strength, durability, and functionality. By studying how atoms arrange themselves and spotting irregularities, you gain insights into improving material properties. Overall, X-ray diffraction provides a powerful tool for understanding the microscopic features that influence macroscopic behavior, making it indispensable in material science and engineering.
Applications of Crystallography in Science and Industry

Crystallography plays an essential role in advancing science and industry by revealing the precise atomic arrangements of materials. You can use it to study crystal growth, helping understand how crystals form and develop in different environments. This knowledge is crucial for improving material properties and manufacturing processes. In industry, crystallography aids mineral identification by analyzing crystal structures to determine mineral composition accurately. This is especially useful in mining, geology, and quality control. Additionally, it helps in developing new materials like pharmaceuticals, where understanding molecular arrangements ensures efficacy and stability. Overall, crystallography enables you to analyze and manipulate the microscopic structures of substances, leading to innovations across various fields and enhancing the quality and performance of products.
Exploring the Beauty of Crystal Structures

The intricate beauty of crystal structures reveals itself through their symmetrical patterns and shimmering facets, mesmerizing both scientists and enthusiasts alike. When you observe a crystal, you witness the result of precise crystal growth, where atoms align perfectly to form stunning geometries. This natural artistry aids in mineral identification, helping you distinguish minerals based on their unique crystal forms. The symmetry and clarity evoke a sense of wonder and admiration for nature’s design. You might find yourself captivated by the way light interacts with these structures, creating mesmerizing reflections. Exploring crystal structures reveals more than just their appearance; it uncovers the fundamental principles behind their formation. Embrace the elegance of these formations, and let their beauty deepen your appreciation for the science behind minerals.
Frequently Asked Questions
How Does Temperature Affect Crystal Growth?
Temperature effects play a pivotal role in crystal growth, influencing how crystals form and develop. When you lower the temperature, it often slows down the growth rate, resulting in more well-defined crystal morphology. Conversely, higher temperatures can increase growth speed but may lead to irregular shapes. You’ll find that controlling temperature helps you tailor crystal size and quality, making it an essential factor in achieving desired crystal structures.
What Are Common Challenges in Crystal Analysis?
Imagine you’re deciphering a complex puzzle; this mirrors solving phase problems and interpreting diffraction patterns in crystal analysis. You face challenges like incomplete data or overlapping signals, which obscure the crystal’s true structure. You must carefully analyze diffraction images, distinguish noise from meaningful signals, and use advanced techniques to fill in gaps. Persistence helps you uncover the crystal’s secrets, turning raw data into a clear, detailed map of its atomic arrangement.
Can Crystallography Study Non-Solid Materials?
Crystallography mainly focuses on solid materials, but it can also study certain non-solid substances like liquid crystals, which have some ordered structure. However, amorphous materials, lacking long-range order, are difficult to analyze using traditional crystallography methods. Instead, techniques like X-ray scattering or spectroscopy are better suited. So, while crystallography isn’t ideal for all non-solids, it can provide insights into some structured liquids and semi-ordered materials.
How Do Defects Influence Crystal Properties?
Defects like point defects and dislocation dynamics markedly influence crystal properties. When you introduce point defects, they disrupt the regular atomic arrangement, affecting electrical and mechanical behaviors. Dislocation dynamics control how the crystal deforms under stress, impacting strength and ductility. By understanding these defects, you can manipulate and improve material performance, making crystals more resilient or conductive depending on your applications.
What Future Advancements Are Expected in Crystallography?
Imagine revealing the universe’s hidden blueprint – that’s what future crystallography promises. You’ll see breakthroughs through innovative imaging techniques that reveal atomic details like never before, while computational modeling accelerates discoveries by simulating crystal behaviors. These advancements will deepen your understanding of materials, paving the way for stronger, lighter, and more sustainable innovations. As technology advances, your ability to manipulate crystals at the atomic level will transform countless fields, from medicine to electronics.
Conclusion
By delving into crystallography, you gently uncover the hidden harmony of the natural world. It’s like peering into nature’s secret dance, where atoms waltz in perfect rhythm. With each discovery, you appreciate the quiet elegance that shapes everything from minerals to medicines. So, keep exploring—there’s a subtle beauty in understanding these intricate structures that’s waiting to inspire your curiosity and spark your imagination.