Mass spectrometry works by converting molecules into charged particles through ionization, then separating these ions based on their mass-to-charge ratio using a mass analyzer. You’ll find components like the ion source, analyzer, detector, and vacuum system working together in a streamlined workflow. Different ionization methods and analyzers suit various samples and analysis goals. To understand the detailed principles and how each part connects, keep exploring—there’s much more to discover.
Key Takeaways
- Mass spectrometry analyzes molecules by ionizing them, separating ions based on their mass-to-charge ratio (m/z), and detecting the resulting ions.
- Key components include the ion source, mass analyzer, detector, inlet system, vacuum environment, and data processing software.
- Different ionization methods (e.g., ESI, MALDI, EI) are used depending on sample type and analysis needs.
- Common mass analyzers—quadrupole, TOF, ion trap, Orbitrap—separate ions with varying resolution and speed.
- MS is widely used across fields like biochemistry, environmental science, forensics, and space exploration for molecule identification and quantification.
Core Principles and Workflow of Mass Spectrometry

Have you ever wondered how mass spectrometry identifies and analyzes molecules? The process starts with preparing your sample in liquid or gas form, ensuring it’s suitable for ionization.
Depending on your molecules, you’ll choose an ionization method—electrospray for biomolecules or electron impact for small compounds—that converts neutral molecules into charged ions. These ions are generated through protonation or electron bombardment, allowing electromagnetic fields to manipulate them. The resulting ions are then directed into the mass analyzer, which separates them based on their mass-to-charge ratio (m/z)—a fundamental parameter in differentiating ions during analysis. The mass analyzer plays a crucial role in determining the resolution and accuracy of the measurement, influencing the overall quality of the data obtained. Detectors then amplify these signals, converting them into digital spectra.
Throughout this workflow, software helps interpret the data, matching observed ions to known databases, enabling you to identify and quantify molecules with high precision. Advances in instrument sensitivity have further enhanced the ability to detect low-abundance analytes accurately, especially when combined with predictive analytics for data analysis. Additionally, understanding the resources and tools available can optimize the entire mass spectrometry process, from sample preparation to data interpretation.
This integration allows for more accurate and comprehensive results, making mass spectrometry an indispensable tool in modern analytical science.
Key Components of a Mass Spectrometer
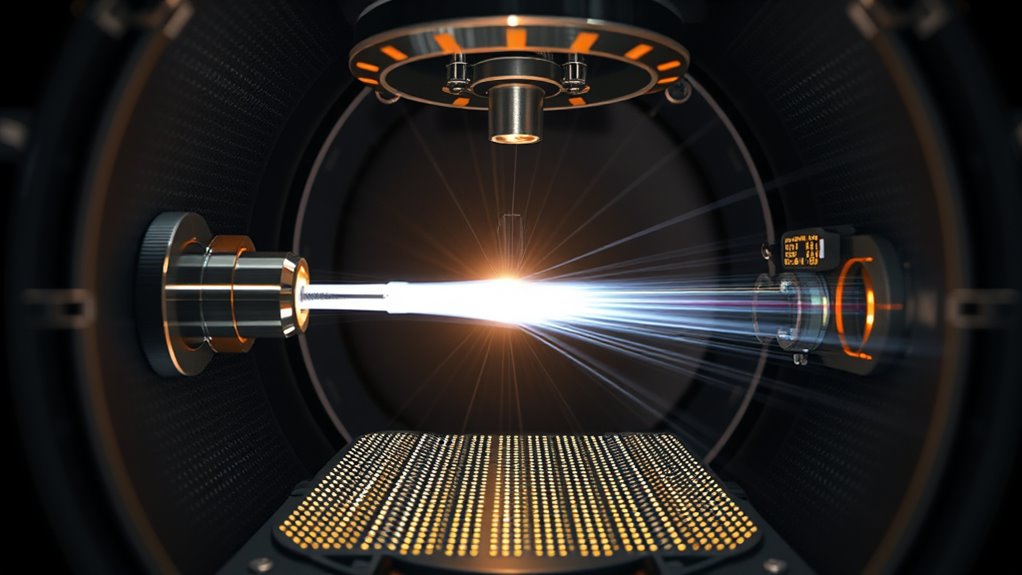
Understanding the key components of a mass spectrometer helps clarify how it analyzes molecules with such precision. First, the ion source converts molecules into ions, using methods like electron ionization, electrospray, or MALDI, depending on your sample. These ions then enter the mass analyzer, where they’re separated based on their mass-to-charge ratios; options include quadrupole, TOF, or Orbitrap analyzers. The detector captures these ions, converting their signals into measurable data—electron multipliers or microchannel plates often amplify the signals. The inlet system introduces your sample, and the entire setup operates under a vacuum to guarantee accuracy. The vacuum system is crucial for maintaining the integrity of the analysis, ensuring that ions are not scattered or lost before detection. Maintaining a controlled environment within the instrument is essential for precise measurements and consistent results. Additionally, sample ionization techniques are vital for converting neutral molecules into charged particles suitable for mass analysis. This process often relies on ionization efficiency, which affects the sensitivity and accuracy of the measurements. Properly managing the filtering process is essential to prevent contamination and maintain the instrument’s performance. A calibrated system is also necessary to ensure that the measurements are accurate over time. Finally, the data processing system interprets the signals, providing you with detailed mass spectra that reveal molecular structures and compositions.
Common Ionization Techniques in Mass Spectrometry

Different ionization techniques in mass spectrometry are tailored to analyze various types of samples, ranging from small volatile molecules to large biomolecules. Electron Ionization (EI) works well with volatile, nonpolar compounds, often in GC-MS setups, producing extensive fragmentation. EI’s ability to generate characteristic fragmentation patterns is beneficial for compound identification, especially when combined with spectral libraries. Chemical Ionization (CI) uses reagent gases to generate softer ionization, ideal for qualitative and quantitative analysis. Atmospheric Pressure Chemical Ionization (APCI) forms protonated molecules, making it compatible with liquid chromatography, and minimizes fragmentation. APCI also offers advantages for analyzing less polar analytes, broadening its applicability. Electrospray Ionization (ESI) suits polar, high-molecular-weight compounds, especially in LC-MS applications. ESI is particularly useful for analyzing complex biological mixtures with minimal fragmentation, preserving the molecular structure of large biomolecules. Matrix-Assisted Laser Desorption/Ionization (MALDI) efficiently ionizes large biomolecules like proteins and peptides. MALDI’s soft ionization is ideal for large, fragile molecules, facilitating their analysis without significant fragmentation. These soft ionization methods preserve molecular integrity, enabling precise identification, while gas-phase techniques like EI and CI are suited for smaller molecules. Advancements in mass spectrometry, such as coupling ionization techniques with high-resolution analyzers, have significantly enhanced detection accuracy and the analysis of complex mixtures.
Types of Mass Analyzers and Their Functions

Mass analyzers are the core components that separate ions based on their mass-to-charge ratios, enabling accurate identification and quantification in mass spectrometry. Quadrupole analyzers filter ions with oscillating electrical fields, offering rapid scanning and cost-effective, routine analysis, though they’ve limited resolution. Ion traps trap ions in 3D space, providing high sensitivity and multi-stage fragmentation, ideal for structural studies but with a narrower dynamic range. TOF analyzers measure ion flight times, accommodating a wide m/z range with high resolution, suitable for proteomics and untargeted analyses, though they need pulsed sources. Orbitrap analyzers trap ions electrostatically, offering ultrahigh resolution and accuracy, perfect for high-precision work like proteomics. Each analyzer type serves specific analytical needs, balancing speed, resolution, and sensitivity. Additionally, understanding the mass-to-charge ratio is essential for selecting the appropriate analyzer based on the analytical requirements. Regular maintenance and cleaning of the analyzers help prevent contamination and ensure optimal performance over time.
Practical Applications of Mass Spectrometry

Mass spectrometry has become an essential tool across various fields by enabling precise analysis of complex samples. In biotechnology, you use MS to study proteins, their interactions, and identify biomarkers that distinguish healthy from diseased states, aiding diagnostics. It also helps in protein sequencing and analyzing drug structures, supporting therapeutic development. Furthermore, MS allows for high-throughput screening, significantly accelerating research processes in drug discovery and development. In the food industry, MS ensures product quality, detects pesticide residues, allergens, and contaminants, and verifies water purity. Environmental scientists rely on MS to assess soil pollutants, analyze atmospheric gases, and monitor water safety. In forensic science, you employ MS to analyze crime scene evidence, toxicology samples, and explosive residues, assisting investigations. Emerging fields like astronomy utilize MS to analyze planetary atmospheres, solar wind, and extraterrestrial geological samples, expanding our understanding of the universe. Additionally, MS can detect antioxidants and vitamins, aiding in nutritional analysis and quality control. Incorporating pimple patch technology into skincare research exemplifies how targeted applications can improve skin health, demonstrating MS’s versatility in product development. A deeper understanding of sample preparation techniques enhances the accuracy and sensitivity of mass spectrometry analyses across all applications.
Historical Development and Technological Advances

The development of mass spectrometry has been shaped by a series of scientific discoveries and technological innovations over the past century. Early research on gas discharges identified ions and electrons, while Prout’s 1815 atomic weight hypothesis influenced early chemistry before being disproved.
The discovery of isotopes with mass spectrometry, starting with neon, proved the method’s ability to differentiate atomic species. J.J. Thomson’s 1910 measurement of a molecular mass spectrum marked a milestone.
Instrumentation evolved with devices like the double-focusing spectrometer (1950) and quadrupole mass spectrometer (1953), improving resolution and ion trapping. Technological advances such as electrospray ionization, tandem MS, and gas chromatography-MS expanded applications.
Key figures like Thomson, Wolfgang Paul, and William Stephens drove innovations, making MS a essential tool across scientific disciplines.
Future Directions and Emerging Trends
As technological innovations continue to accelerate, the future of mass spectrometry is set to transform various industries through miniaturization, enhanced analytical capabilities, and smarter data processing. You’ll see portable devices that enable on-site analysis, making testing faster and more accessible. The global market is projected to reach over USD 10 billion by 2029, driven by increasing demand in food safety, environmental monitoring, and healthcare, with projections reaching over $10 billion by 2029. Advances in AI and machine learning will improve data interpretation, allowing for more accurate predictions and insights. Novel ionization techniques like ambient ionization and improved analyzers such as Orbitrap and FT-ICR will boost resolution and sensitivity. Market growth is driven by increasing demand in food safety, environmental monitoring, and healthcare, with projections reaching over $10 billion by 2029. Cross-industry collaborations and government investments will further expand applications, particularly in precision medicine, proteomics, and metabolomics, shaping a more efficient, versatile future for mass spectrometry.
Frequently Asked Questions
How Does Mass Spectrometry Differentiate Isobaric Compounds?
You differentiate isobaric compounds by using advanced mass spectrometry techniques that exploit subtle differences. For example, ion-mobility mass spectrometry separates ions based on their shape and size.
While PTR-MS uses chemical reactions to distinguish their spectral features. Tandem MS (MS/MS) analyzes fragmentation patterns, and gas-phase ion reactions provide additional specificity. These methods help you accurately identify and differentiate compounds even when their masses are identical.
What Are the Limitations of Current Mass Spectrometry Techniques?
The limitations of current mass spectrometry techniques are like trying to see through a foggy window. You might miss low-abundance metabolites, struggle with complex samples, or face resolution and dynamic range issues.
Sample heterogeneity and ion suppression can distort results, while high costs and the need for specialized skills slow progress.
These challenges hinder rapid, accurate analysis, making it tough to fully access the potential of mass spectrometry in diverse research and clinical applications.
How Is Quantitative Accuracy Achieved in Mass Spectrometry?
You achieve quantitative accuracy in mass spectrometry by using internal standards like isotope-labeled compounds to correct for ionization efficiency variations. Calibration curves with known analyte concentrations help guarantee linearity.
Techniques like SRM/MRM mode monitor specific precursor-product ion passage, while matrix-matched calibration minimizes ion suppression.
Validating the limit of quantification and performing regular calibration and quality control checks keep your measurements precise and reliable.
Can Mass Spectrometry Analyze Non-Gaseous or Solid Samples Directly?
Think of mass spectrometry as a versatile detective, capable of uncovering secrets hidden within solids and non-gaseous samples.
You can analyze these samples directly using advanced ionization techniques like MALDI, DART, DESI, and SIMS, which act like magic wands, transforming solids into detectable ions without dissolving them.
These methods bypass traditional barriers, allowing you to probe surface compositions, elemental makeup, or molecular structures in their native form.
What Safety Precautions Are Necessary When Operating Mass Spectrometers?
When operating mass spectrometers, you need to follow essential safety precautions. Always inspect the instrument for proper function, avoid touching hot parts, and let it cool before maintenance.
Be aware of high voltage areas, never bypass safety shields, and ensure only qualified technicians perform repairs.
Wear PPE, handle chemicals carefully, and operate in well-ventilated areas.
Follow all warning labels, and handle samples in appropriate safety environments to prevent accidents.
Conclusion
Understanding mass spectrometry reveals powerful insights into molecular structures, vital for research and industry. Did you know that over 70% of pharmaceutical discoveries rely on this technique? As technology advances, your ability to analyze complex compounds becomes even more precise. Embrace these innovations, and you’ll stay at the forefront of scientific discovery, making impactful contributions. Mass spectrometry isn’t just a tool; it’s your gateway to uncovering the secrets of matter.









